Study setting
We conducted an observational cohort study derived from an ongoing, prospective, multicenter surveillance network REA-REZO of ICU-acquired infections and associated risk factors. This cohort included individual patient data from 206…
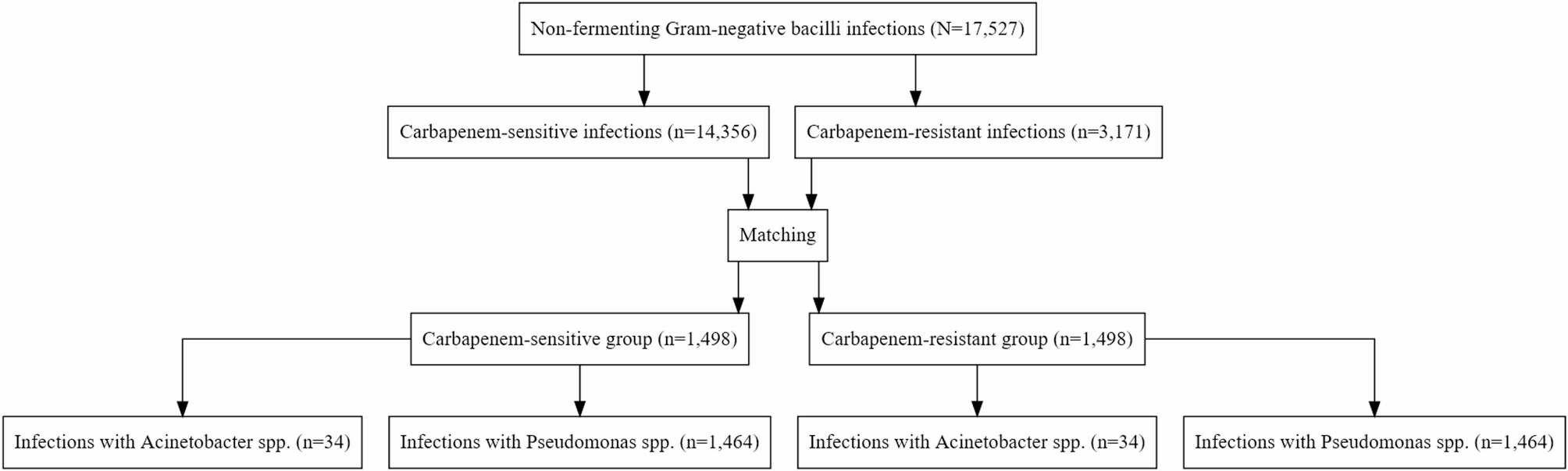
We conducted an observational cohort study derived from an ongoing, prospective, multicenter surveillance network REA-REZO of ICU-acquired infections and associated risk factors. This cohort included individual patient data from 206…

The average age of PGDM mothers participating in the study was 31.72 ± 5.17, with 77% having a high school education or higher, 57.5% unemployed, 22.5% smoking, 25% having a chronic illness, and experiencing the most common complications…
A research team from the University of Zurich and the University Children’s Hospital Zurich has developed a new approach for treating children with malignant tumors of the nervous system. By combining an approved drug with a…
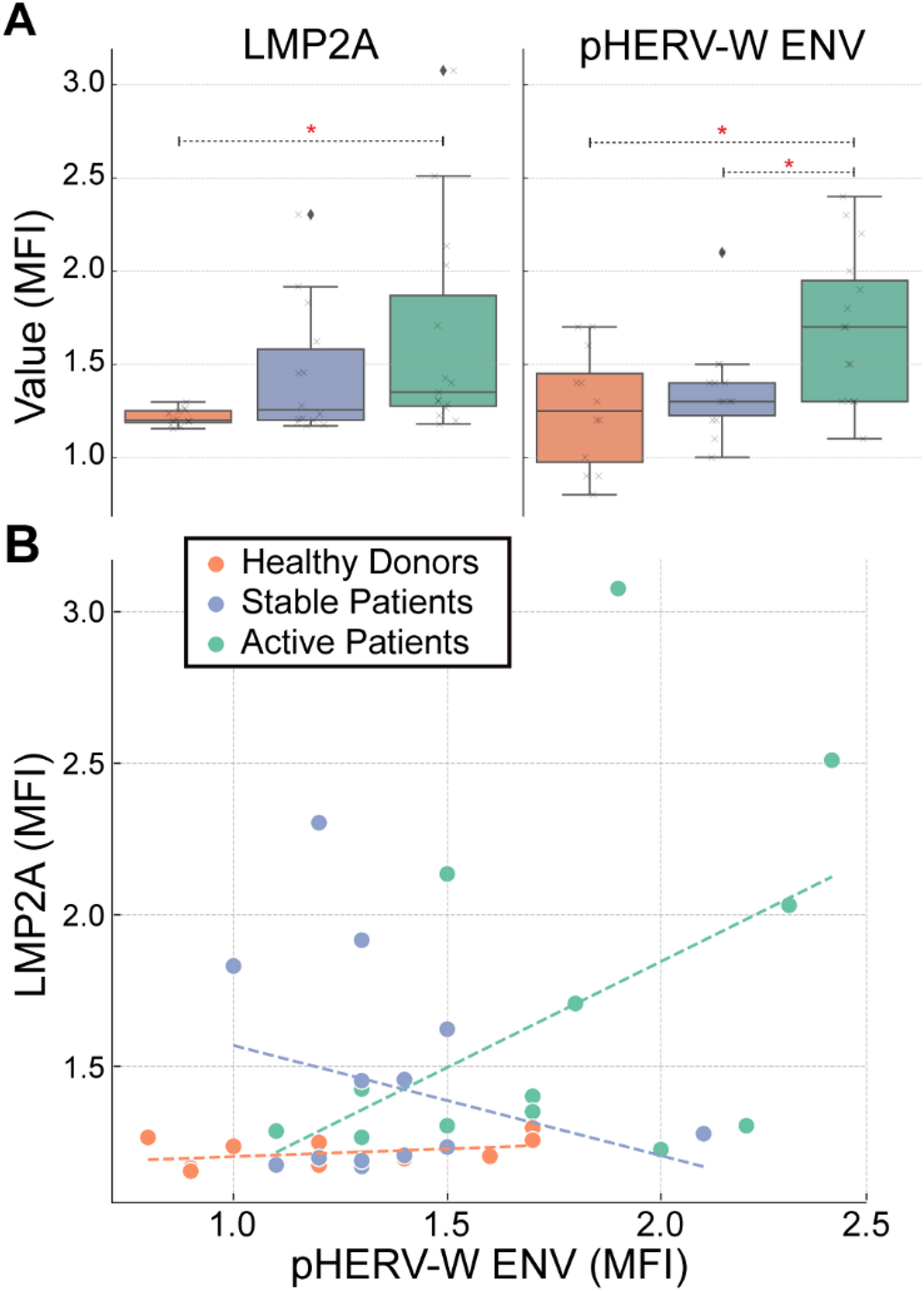
Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. 2019;26(1):27–40. https://doi.org/10.1111/ene.13819.
Google Scholar
Lanz TV, Brewer RC, Ho PP, Moon JS, Jude KM, Fernandez D, et al. Clonally expanded B cells in multiple sclerosis bind EBV EBNA1 and glialcam. Nature. 2022;603(7900):321–7. https://doi.org/10.1038/s41586-022-04432-7.
Google Scholar
Bjornevik K, Münz C, Cohen JI, Ascherio A. Epstein–Barr virus as a leading cause of multiple sclerosis: mechanisms and implications. Nat Rev Neurol. 2023;19(3):160–71. https://doi.org/10.1038/s41582-023-00775-5.
Google Scholar
Morandi E, Tanasescu R, Tarlinton RE, Constantinescu CS, Zhang W, Tench C, et al. The association between human endogenous retroviruses and multiple sclerosis: A systematic review and meta-analysis. PLoS ONE. 2017;12(2):e0172415. https://doi.org/10.1371/journal.pone.
Google Scholar
Küry P, Nath A, Créange A, Dolei A, Marche P, Gold J, et al. Human endogenous retroviruses in neurological diseases. Trends Mol Med. 2018;24(4):379–94. https://doi.org/10.1016/j.molmed.2018.02.007.
Google Scholar
Nevalainen T, Autio-Kimura A, Hurme M. Human endogenous retrovirus W in multiple sclerosis: transcriptional activity is associated with decline in oligodendrocyte proportions in the white matter of the brain. J Neurovirol. 2024;30(4):393–405. https://doi.org/10.1007/s13365-024-01208-9.
Google Scholar
Gruchot J, Reiche L, Werner L, Herrero F, Schira-Heinen J, Meyer U, et al. Molecular dissection of HERV-W dependent microglial- and astroglial cell polarization. Microbes Infect. 2025;27(5–6):105382. https://doi.org/10.1016/j.micinf.2024.
Google Scholar
Kremer D, Gruchot J, Weyers V, Oldemeier L, Gottle P, Healy L, et al. PHerv-w envelope protein fuels microglial cell-dependent damage of myelinated axons in multiple sclerosis. Proc Natl Acad Sci U S A. 2019;116(30):15216–25. https://doi.org/10.1073/pnas.1901283116.
Google Scholar
Gruchot J, Lewen I, Dietrich M, Reiche L, Sindi M, Hecker C, et al. Transgenic expression of the HERV-W envelope protein leads to polarized glial cell populations and a neurodegenerative environment. Proc Natl Acad Sci U S A. 2023;120(38):e2308187120. https://doi.org/10.1073/pnas.
Google Scholar
Charvet B, Pierquin J, Brunel J, Gorter R, Quetard C, Horvat B, et al. Human endogenous retrovirus type W envelope from multiple sclerosis demyelinating lesions shows unique solubility and antigenic characteristics. Virol Sin. 2021;36(5):1006–26. 10.7/s12250-021-00372-0.
Google Scholar
Perron H, Germi R, Bernard C, Garcia-Montojo M, Deluen C, Farinelli L, et al. Human endogenous retrovirus type W envelope expression in blood and brain cells provides new insights into multiple sclerosis disease. Mult Scler. 2012;18(12):1721–36. 10.177/1352458512441381.
Google Scholar
Ruberto S, Domınguez-Mozo MI, Garcıa-Martınez MA, Cossu D, Sechi LA, Alvarez-Lafuente R. Immune response profiling of HERV-W envelope proteins in multiple sclerosis: potential biomarkers for disease progression. Front Immunol. 2025;15:1505239. https://doi.org/10.3389/fimmu.2024.
Google Scholar
Mameli G, Poddighe L, Mei A, Uleri E, Sotgiu S, Serra C, et al. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: inference for multiple sclerosis. PLoS ONE. 2012;7(9):e44991. https://doi.org/10.1371/journal.pone.0044991.
Google Scholar
Mameli G, Madeddu G, Mei A, Uleri E, Poddighe L, Delogu LG, et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: the missing link with multiple sclerosis? PLoS ONE. 2013;8(11):e78474. https://doi.org/10.1371/journal.pone.0078474.
Google Scholar
Ebrahimkhani S, Vafaee F, Young PE, Hur SSJ, Hawke S, Devenney E, et al. Exosomal microrna signatures in multiple sclerosis reflect disease status. Sci Rep. 2017;7(1):14293. https://doi.org/10.1038/s41598-017-14301-3.
Google Scholar
Palazzo C, Asci I, Russo S, Buccoliero C, Mangialardi V, Abbrescia P, et al. Circulating exosomes with unique lipid signature in relapsing remitting multiple sclerosis. Front Cell Neurosci. 2025;19:1613618. https://doi.org/10.3389/fncel.2025.
Google Scholar
D’Anca M, Fenoglio C, Buccellato FR, Visconte C, Galimberti D, Scarpini E. Extracellular vesicles in multiple sclerosis: role in the pathogenesis and potential usefulness as biomarkers and therapeutic tools. Cells. 2021;10(7):1733. https://doi.org/10.3390/cells10071733.
Google Scholar
Teow SY, Liew K, Khoo AS, Peh SC. Pathogenic role of exosomes in Epstein-Barr virus (EBV)-associated cancers. Int J Biol Sci. 2017;13(10):1276–86. https://doi.org/10.7150/ijbs.19531.
Google Scholar
Meier UC, Cipian RC, Karimi A, Ramasamy R, Middeldorp JM. Cumulative roles for Epstein-Barr virus, human endogenous retroviruses, and human herpes virus-6 in driving an inflammatory cascade underlying MS pathogenesis. Front Immunol. 2021;12:757302. https://doi.org/10.3389/fimmu.2021.
Google Scholar
Mrad MF, Saba ES, Nakib L, Khoury SJ. Exosomes from subjects with multiple sclerosis express EBV-derived proteins and activate monocyte-derived macrophages. Neurol Neuroimmunol Neuroinflamm. 2021;8(4):e1004. 10.212/NXI.0000000000001004.
Google Scholar
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–73. https://doi.org/10.1016/S474-4422(17)30470-2.
Google Scholar
Kremer D, Forster M, Schichel T, Gottle P, Hartung HP, Perron H, et al. The neutralizing antibody GNbAC1 abrogates HERV-W envelope protein-mediated oligodendroglial maturation Blockade. Mult Scler. 2015;21(9):1200–3. 10.177/1352458514560926.
Google Scholar
Hartung HP, Derfuss T, Cree BA, Sormani MP, Selmaj K, Stutters J, et al. Efficacy and safety of Temelimab in multiple sclerosis: results of a randomized phase 2b and extension study. Mult Scler. 2022;28(3):429–40. https://doi.org/10.1177/13524585211024997.
Google Scholar
Curtin F, Champion B, Davoren P, Duke S, Ekinci EI, Gilfillan C, et al. A safety and pharmacodynamics study of temelimab, an antipathogenic human endogenous retrovirus type W envelope monoclonal antibody, in patients with type 1 diabetes. Diabetes Obes Metab. 2020;22(7):1111–21. 10./dom.14010.
Google Scholar
Simula ER, Jasemi S, Cossu D, Fais M, Cossu I, Chessa V, et al. Human endogenous retroviruses as novel therapeutic targets in neurodegenerative disorders. Vaccines. 2025;13(4):415. https://doi.org/10.3390/vaccines13040415.
Google Scholar
Chen J, Foroozesh M, Qin Z. Transactivation of human endogenous retroviruses by tumor viruses and their functions in virus-associated malignancies. Oncogenesis. 2019;8(1):6. https://doi.org/10.1038/s41389-018-0114-y.
Google Scholar
Brütting C, Stangl G, Staege M, Vitamin D. Epstein-Barr virus, and endogenous retroviruses in multiple sclerosis – facts and hypotheses. J Integr Neurosci. 2021;20(1):233–8. https://doi.org/10.31083/j.jin.2021.01.392.
Google Scholar
Latifi T, Zebardast A, Marashi SM. The role of human endogenous retroviruses (HERVs) in multiple sclerosis and the plausible interplay between HERVs, Epstein-Barr virus infection, and vitamin D. Mult Scler Relat Disord. 2022;57:103318. https://doi.org/10.1016/j.msard.2021.
Google Scholar
Gottle P, Schichel K, Reiche L, Werner L, Zink A, Prigione A, et al. TLR4 associated signaling disrupters as a new means to overcome HERV-W envelope-mediated myelination deficits. Front Cell Neurosci. 2021;15:777542. https://doi.org/10.3389/fncel.2021.
Google Scholar
Osaid Z, Haider M, Hamoudi R, Harati R. Exosomes interactions with the blood–brain barrier: implications for cerebral disorders and therapeutics. Int J Mol Sci. 2023;24(21):15635. https://doi.org/10.3390/ijms242115635.
Google Scholar
Gimenez-Orenga K, Oltra E. Human endogenous retrovirus as therapeutic targets in neurologic disease. Pharmaceuticals (Basel). 2021;14(6):495. https://doi.org/10.3390/ph14060495.
Google Scholar
Adler GL, Le K, Fu Y, Kim WS. Human endogenous retroviruses in neurodegenerative diseases. Genes. 2024;15(6):745. https://doi.org/10.3390/genes15060745.
Google Scholar
Bhetariya PJ, Kriesel JD, Fischer KF. Analysis of human endogenous retrovirus expression in multiple sclerosis plaques. J Emerg Dis Virol. 2017;3(2). https://doi.org/10.16966/2473-1846.133.
Moyes DL, Goris A, Ban M, Compston A, Griffiths DJ, Sawcer S, et al. HERV-K113 is not associated with multiple sclerosis in a large family-based study. AIDS Res Hum Retroviruses. 2008;24(3):363–5. https://doi.org/10.1089/aid.2007.0196.
Google Scholar
Brudek T, Christensen T, Aagaard L, Petersen T, Hansen HJ, Møller-Larsen A. B cells and monocytes from patients with active multiple sclerosis exhibit increased surface expression of both HERV-H Env and HERV-W Env, accompanied by increased seroreactivity. Retrovirology. 2009;6:104. https://doi.org/10.1186/742-4690-6-104.
Google Scholar
Laska MJ, Brudek T, Nissen KK, Christensen T, Moller-Larsen A, Petersen T, et al. Expression of HERV-Fc1, a human endogenous retrovirus, is increased in patients with active multiple sclerosis. J Virol. 2012;86(7):3713–22. https://doi.org/10.1128/JVI.06723-11.
Google Scholar
Censi ST, Mariani-Costantini R, Granzotto A, Tomassini V, Sensi SL. Endogenous retroviruses in multiple sclerosis: A network-based etiopathogenic model. Ageing Res Rev. 2024;99:102392. https://doi.org/10.1016/j.arr.2024.
Google Scholar
Ruberto S, Cossu D, Sechi LA. Correlation between antibodies against the pathogenic pHERV-W envelope protein and the inflammatory phase of multiple sclerosis. Immunology. 2024;171(2):270–6. https://doi.org/10.1111/imm.13712.
Google Scholar
Lyu L, Li Q, Wang C. EBV latency programs: molecular and epigenetic regulation and its role in disease pathogenesis. J Med Virol. 2025;97(7):e70501. https://doi.org/10.1002/jmv.
Google Scholar
Aarts SABM, Seijkens TTP, Dorst KJFv, Dijkstra CD, Kooij G, Lutgens E. The CD40–CD40L dyad in experimental autoimmune encephalomyelitis and multiple sclerosis. Front Immunol. 2017;8:1791. https://doi.org/10.3389/fimmu.2017.01791.
Google Scholar
Vermersch P, Wagner D, Mars LT, Noelle R, Giovannoni G. Inhibiting CD40 ligand in multiple sclerosis: a review of emerging therapeutic potential. Curr Treat Options Neurol. 2025;27:7. https://doi.org/10.1007/s11940-024-00818-2.
Google Scholar
Orian JM, D’Souza CS, Kocovski P, Krippner G, Hale MW, Wang X, et al. Platelets in multiple sclerosis: early and central mediators of inflammation and neurodegeneration and attractive targets for molecular imaging and site-directed therapy. Front Immunol. 2021;12:620963. https://doi.org/10.3389/fimmu.2021.
Google Scholar
Burnouf T, Walker TL. The multifaceted role of platelets in mediating brain function. Blood. 2022;140(8):815–27. https://doi.org/10.1182/blood.2022015970.
Google Scholar
Brudek T, Christensen T, Petersen T, Møller-Larsen A. Expression of HERV-H/W Env epitopes on PBMCs from MS patients with active disease. Retrovirology. 2011;8(Suppl 1):A210. https://doi.org/10.1186/742-4690-8-S1-A210.
Google Scholar
Chunder R, Schropp V, Kuerten S. B cells in multiple sclerosis and virus-induced neuroinflammation. Front Neurol. 2020;11:591894. https://doi.org/10.3389/fneur.2020.
Google Scholar
Garcia-Montojo M, Rodríguez-Martín E, Ramos-Mozo P, Ortega-Madueño I, Domínguez-Mozo M, Arias-Leal A, et al. Syncytin‐1/HERV‐W envelope is an early activation marker of leukocytes and is upregulated in multiple sclerosis patients. Eur J Immunol. 2020;50(5):685–94. https://doi.org/10.1002/eji.201948423.
Google Scholar
Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of extracellular vesicles across the Blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci. 2020;21(12):4407. https://doi.org/10.3390/ijms21124407.
Google Scholar
Gjelstrup MC, Stilund M, Petersen T, Møller H, Petersen EL, Christensen T. Subsets of activated monocytes and markers of inflammation in incipient and progressed multiple sclerosis. Immunol Cell Biol. 2017;96(2):160–74. https://doi.org/10.1111/imcb.025.
Google Scholar
Ferreira-Atuesta C, Reyes S, Giovanonni G, Gnanapavan S. The evolution of neurofilament light chain in multiple sclerosis. Front Neurosci. 2021;15:642384. https://doi.org/10.3389/fnins.2021.
Google Scholar
Virata MCA, Catahay JA, Lippi G, Henry BM. Neurofilament light chain: a biomarker at the crossroads of clarity and confusion for gene-directed therapies. Neurodegener Dis Manag. 2024;14(6):227–39. https://doi.org/10.1080/17582024.2024.2421738.
Google Scholar
Steffen F, Uphaus T, Ripfel N, Fleischer V, Schraad M, Gonzalez-Escamilla G, et al. Serum neurofilament identifies patients with multiple sclerosis with severe focal axonal damage in a 6-year longitudinal cohort. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):e200055. https://doi.org/10.1212/NXI.0000000000200055.
Google Scholar
Freedman MS, Abdelhak A, Bhutani MK, Freeman J, Gnanapavan S, Hussain S, et al. The role of serum neurofilament light (sNfL) as a biomarker in multiple sclerosis: insights from a systematic review. J Neurol. 2025;272(6):400. https://doi.org/10.1007/s00415-025-13093-1.
Google Scholar
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!
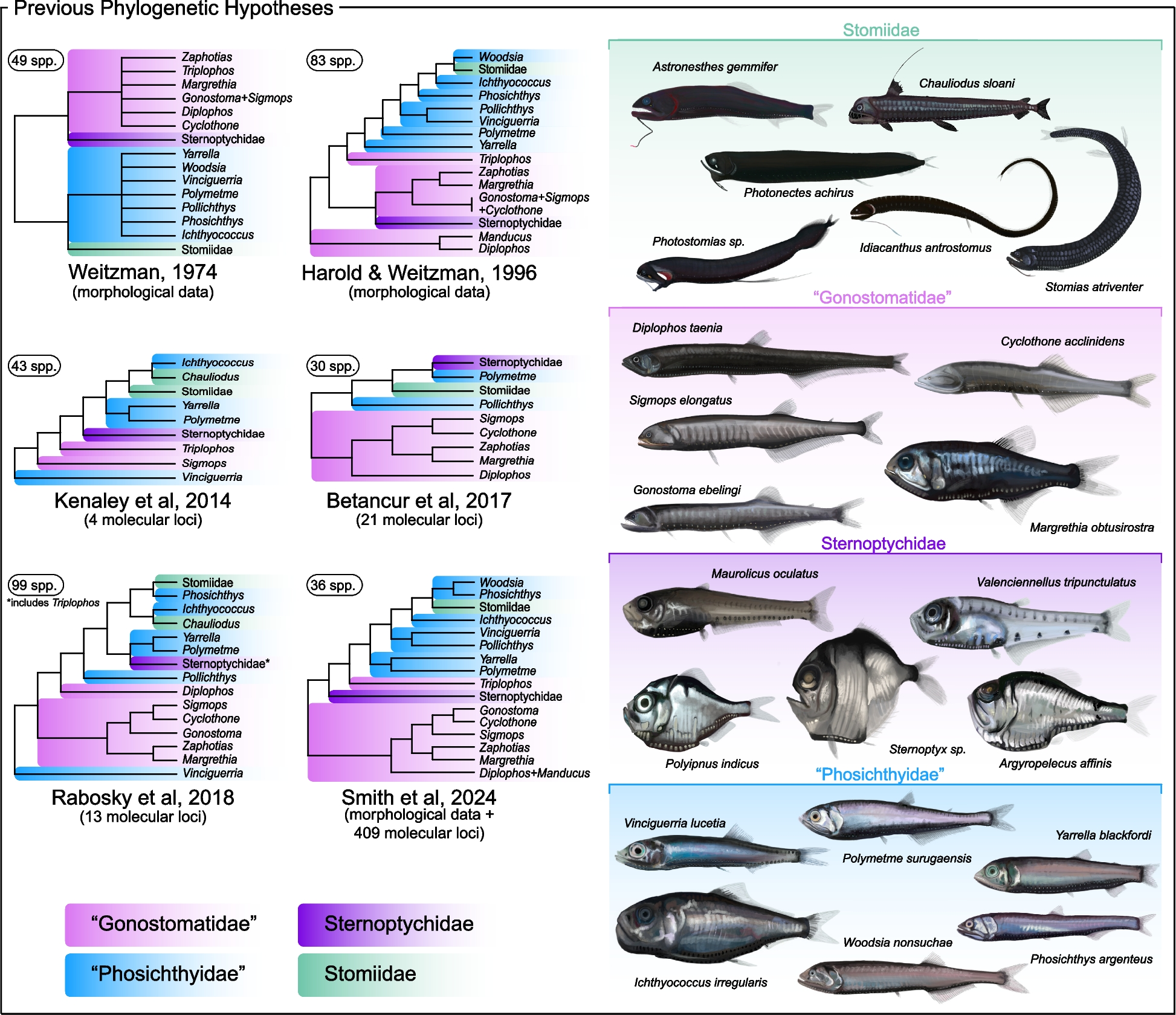
Our primary nuclear exon-based phylogenomic dataset included 60 stomiiform species [31 genera, 4 families] and four outgroup taxa, comprising 936 loci totaling 314,607 base pairs (bp),…
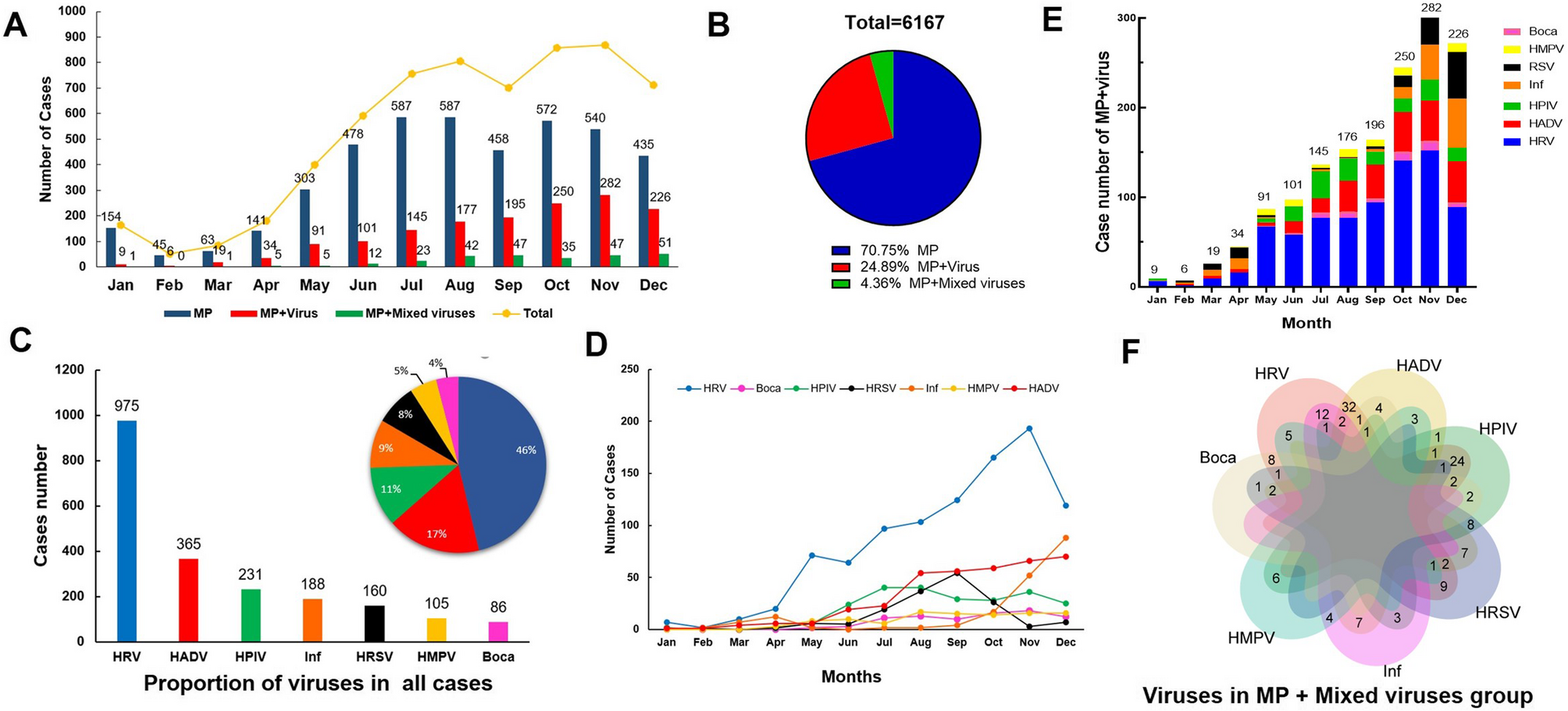
During the study period from Jan, 2023 to Dec, 2023, a total of 14,907 patients with CAP were initially included in this study, following the exclusion criteria, 6,167 pediatric patients were ultimately enrolled. Of these, 4347…
Nine male patients were enrolled during January 2019 to September 2019. Due to the COVID-19 pandemic, study participation was incomplete. Pre-COVID pandemic onset (March 2020), 80.1% of the expected 171 visits were attended (including baseline visits). Post-COVID pandemic, 19.7% of the 458 scheduled visits were attended. The cognitive assessments DAS-II and VABS-3 were performed at baseline only. All other assessments had follow-up visits completed at varying time points (Supplementary Table 1).
We included eight pediatric males and one adult male from the USA, comprising eight families. The characteristics of the study population are summarized in Table 1. Nonsense mutations occurred in six patients (66%) and three patients (33%) had deletions (Table 2).
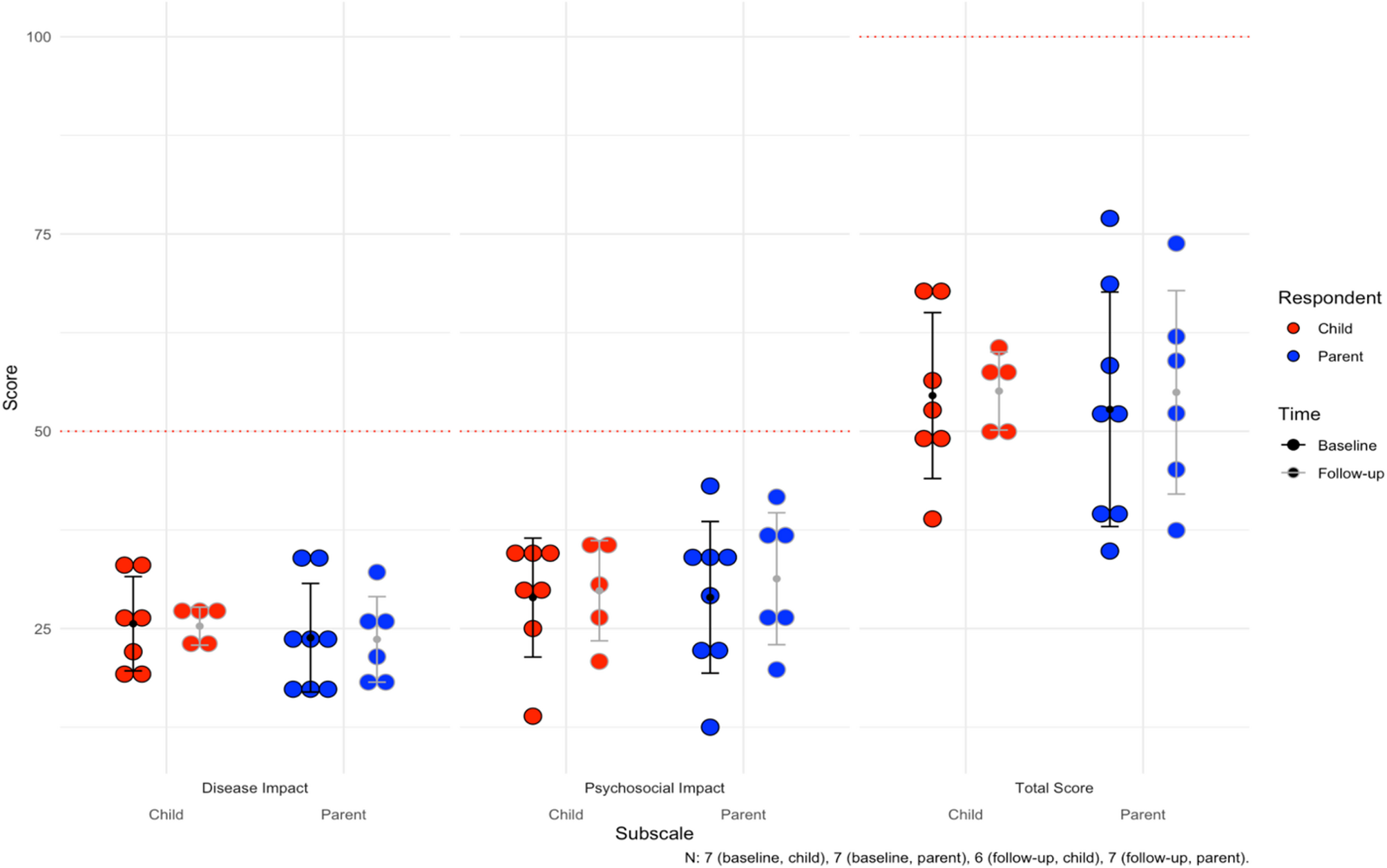
Seven of eight pediatric patients had baseline PCQLI responses (88%, n = 7), of which seven had follow-up responses (100%, n = 7). The baseline mean for the Disease Impact subscale was 25.6 ± 6.0. The mean follow-up time was 26.6 months for both patient and parent responses (see all responses in Supplementary Table 2). At last follow up, four patients (57%, n = 4) reported lower Disease Impact scores. For the Psychosocial Impact Score, the mean at baseline was 28.9 ± 7.5. At last follow-up, three patients (43%, n = 3) reported lower scores. For the Total Score, the mean was 54.5 ± 10.5 at baseline. At last follow-up, four patients (57%, n = 4) reported lower total scores.
For the parents, there were eight baseline responses (100%, n = 8), of which seven had follow-up responses (88%, n = 7). Mean follow-up time was 26.6 months. The baseline mean for Disease Impact was 23.8 ± 6.9, with three parents (43%, n = 3) reporting lower scores for their children at last follow up. Mean Psychosocial Impact score at baseline was 28.9 ± 9.6, with five parents (71%, n = 5) reporting lower scores at last follow-up. Finally, the mean Total Score was 52.8 ± 14.9 at baseline, and four parents (57%, n = 4) reported lower total scores for their children at last follow up. The mean scores can be visualized for children and parents in Fig. 1. Notably, average difference between patient and parent Total Scores were 9.1 ± 7.9 and concordance in longitudinal improvements or worsening of scores over time between patients and parents was 75% (n = 9).
Average subscale and total scores for the Pediatric Cardiac Quality of Life Inventory (PCQLI) across the cohort. For Disease Impact and Psychosocial Impact subscales, 50 is the maximum score (100 for Total Score). The mean follow-up time is 26.6 months. The error bars are the mean and standard deviations for each subgroup
The average scores for the subscales of the PQLQ can be found in Table 3 (see all responses in Supplementary Table 3). Both patients and their parents responded to this. The max score for all subscales was 100. The mean follow-up time was 28.3 months. Figure 2 shows the scores for physical, emotional, social, and school functioning.
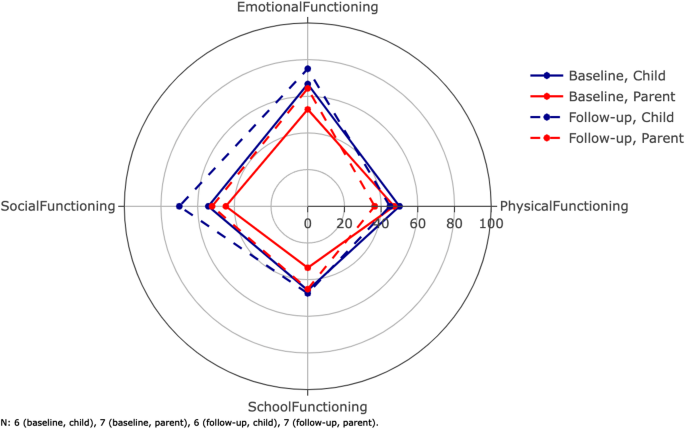
Average scores for the dimensions of the Pediatric Quality of Life Questionnaire (PQLQ). The inventory is filled out by both patients and parents. The parents score their children as functioning worse than the patients personally score themselves. The composite scores total scores are not shown on this radar plot (Table 3). Error bars are not included here in order to maintain visibility. The mean follow-up time was 28.3 months
Six of eight pediatric patients had baseline responses (75%, n = 6), of which five patients had follow-up responses (83%, n = 5). For the physical health summary score, which is equal to the physical functioning score, the baseline mean was 50.0 ± 6.3; two patients (40%, n = 2) reported lower physical health summary scores at follow-up. For the psychosocial health summary score, the baseline mean was 55.3 ± 24.7; one patient (17%, n = 1) reported a lower psychosocial health summary score for themselves at follow-up. For emotional functioning, baseline mean was 66.7 ± 24.8, and two patients (40%, n = 2) reported worse functioning at follow-up (Fig. 2). For social functioning, baseline mean was 54.2 ± 32.9, and four patients (60%, n = 3) reported worse functioning at follow-up. For school functioning, baseline mean was 45.8 ± 30.1, and four patients (60%, n = 3) reported worse functioning at follow-up. Finally, for the total score, baseline mean was 53.4 ± 17.6, and one patient (20%, n = 1) reported a lower total score at follow-up.
Seven parents had baseline responses (88%, n = 7), of which six had follow-up responses (86%, n = 6) (Table 3). For the physical health summary score, the mean at baseline was 47.1 ± 8.4. At follow-up, all parents (100%, n = 7) reported worse functioning in their child. For the psychosocial health summary score, the baseline mean was 43.7 ± 16.0 and only one parent (17%, n = 1) reported a lower follow-up psychosocial health summary score. For emotional functioning, baseline mean was 52.9 ± 30.4, and four parents (67%, n = 4) reported worse functioning at follow-up. For social functioning, baseline mean was 44.6 ± 13.7, and four parents (67%, n = 4) reported worse functioning at follow-up. For school functioning, baseline mean was 33.6 ± 9.4, and three patients (50%, n = 3) reported worse functioning at follow-up. Finally, for the total score, baseline mean was 44.9 ± 8.6, and four parents (67%, n = 4) reported lower total scores at follow-up. Average difference between patient and parent total scores was 13.4 ± 9.1. Additionally, concordance in longitudinal changes in patient and parent total scores was 58% (n = 5). Parents were more likely to describe physical decline at follow up compared to the patients themselves. Although the remaining emotional, social and school functioning dimensions were more concordant between parents and patients, the total PQLQ parent scores were still lower at follow up compared to patients.
In our cohort, all patients (100%, n = 9) were affected by neuropsychological symptoms that included: learning difficulties (100%, n = 9), attention-deficit hyperactivity disorder (56%, n = 5), anxiety (44%, n = 4), depression (44%, n = 4), autism (22%, n = 2) and global developmental delay (11%, n = 1).
Six patients in the cohort completed the DAS-II at baseline. Figure 3a shows the average score for our cohort in each of the domains (see the scores for each domain for each patient in Supplementary Table 4). The mean at baseline for each domain in our cohort was: GCA, 67.0 ± 14.7; verbal, 66.3 ± 19.0; nonverbal, 70.5 ± 18.3; spatial, 68.7 ± 18.3.
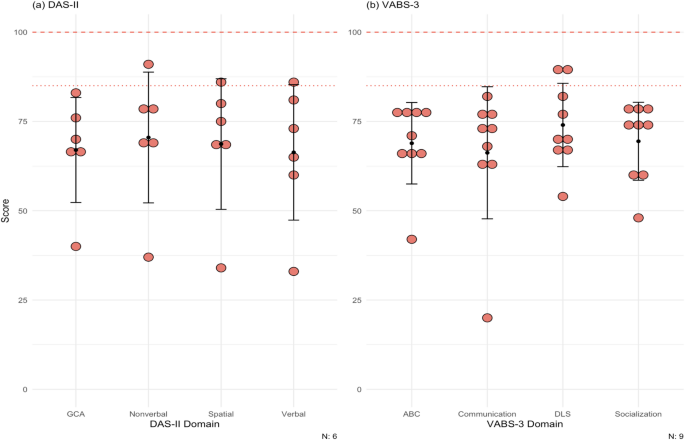
(a) Scores for patients for the DAS-II. Population mean and standard deviation for the domains tested are 100 (dashed line) ± 15 (dotted line). Patient 6, 8, 9 did not complete the DAS-II. Most DD patients lie below at least 1 standard deviation on both cognitive tests. (b) Scores for patients for the VABS-3. Population means and standard deviations for the domains tested are 100 (dashed line) ± 15 (dotted line). All patients completed this test at baseline only. The maximum score of the test is 170. Most of the patients scored below at least 1 standard deviation on all domains. DAS-II = Differential Ability Scales Second Edition, VABS-3 = Vineland Adaptive Behavior Scales Third Edition, GCA = General Conceptual Ability, ABC = Adaptive Behavior Composite, DLS = daily living skills
All patients completed the VABS-3 at baseline. The mean at baseline for each domain in our cohort was: ABC, 68.9 ± 11.4; communication, 66.2 ± 18.5; daily living skills 74.0 ± 11.7; socialization, 69.4 ± 10.9 (Fig. 3b; see the scores for each domain for each patient in Supplementary Table 5).
DD patients very commonly have visual disturbances. In our cohort, eight patients had one or more ophthalmological changes (89%, n = 8): nyctalopia (11%, n = 1), photophobia (11%, n = 1), blurry vision (33%, n = 3), myopia (78%, n = 7), prescribed corrective lenses (78%, n = 7), and photophobia (89%, n = 8). The BCVA was within the normal range for all patients (100%, n = 9). Of note, hyperreflective foci were identified at the level of the outer nuclear layer (ONL) and the border of the outer plexiform layer in six patients (67%, n = 6) in SD-OCT (example in Fig. 4). These patients also had paler fundus pseudocolor images with what appeared to be a loss of retinal pigmentation. Fundus autofluorescence imaging demonstrated increased macular autofluorescence.
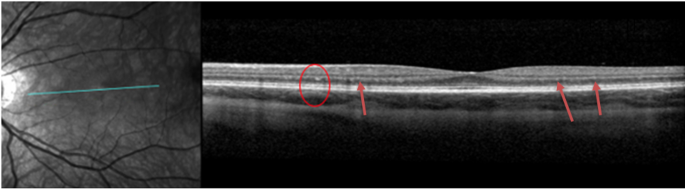
Spectral Domain Optical Coherence Tomography (SD-OCT) of the left eye of patient 5 showing hyper-reflective foci on the border of the outer nuclear layer (ONL) foci and outer plexiform layer (OPL) (red circle and red arrows)
Most patients in the cohort reported cardiac related symptoms (78%, n = 7): dyspnea (33%, n = 3), palpitations (44%, n = 4), and chest pain (56%, n = 5). All nine patients had baseline echocardiograms (100%, n = 9) and seven patients had follow-up echocardiograms (78%, n = 7). Regarding outcomes, one patient underwent transplantation during the study period at age 14. There were no deaths or ventricular assist device implantations. Two pediatric patients had baseline data only; one patient underwent cardiac transplantation and another patient stopped following up due to the COVID pandemic. Due to standardized z-score reporting in pediatric patients, we separated the pediatric patients (n = 8) from the adult patient (n = 1). The mean follow-up time was 24.5 months for the pediatric patients. Mean EF at baseline was 68.9 ± 4.8%. After the mean follow-up time, all six pediatric patients with follow-ups had lower EF values (100%, n = 6). The mean EF at last follow-up was 64.3 ± 8.5%, with an average paired difference of -5.9% which based on the Wilcoxon signed-rank test, was not statistically significant. The adult patient had a baseline EF of 47% and at last follow-up 41%.
For wall thickness, we utilized a maximum wall thickness (MWT) that measured the largest of the septal or posterior wall. The average MWT at baseline for the pediatric patients was 13.6 ± 7.1 mm (z-score = 6.9 ± 6.2; 75% [n = 6] of pediatric patients had a z-score > 2 at baseline). At last follow-up, wall thickness increased in all six pediatric patients (100%, n = 6). The mean MWT at last follow-up was 15.6 ± 6.8 mm (z-score = 9.4 ± 7.6), with an average paired difference of 3.8 mm, which based on the Wilcoxon signed-rank test, was not statistically signfiicant. For the young adult, his MWT was 30.4 mm at baseline and 28.2 mm at last follow-up after six months.
Mean LVEDD at baseline for the pediatric patients was 37.8 ± 5.7 mm (z-score = -1.6 ± 1.8). At last follow-up, four patients had a smaller LVEDD (67%, n = 4). Both patients with increased LVEDD, dilated in isolation of ventricular wall thinning, but did experience a drop in LVEF. The mean at last follow-up for the pediatric patients was 36.5 ± 6.7 mm (z-score = -2.1 ± 1.5), with an average paired difference of -0.5 mm. For the young adult, who had LV wall thinning and a drop in his LVEF during follow up, his LVEDD was 43.1 mm at baseline and 46.2 mm at last follow-up after six months.
Mean LV mass at baseline for the pediatric patients was 192.7 ± 133.1 g (z-score = 7.5 ± 6.6). Of the pediatric patients in the cohort (n = 8), 75% (n = 6) had a z-score >2 at baseline. At follow-up (n = 6), six patients had increased LV mass (100%, n = 6). Mean LV mass at last follow-up was 200.7 ± 88.9 g (z-score = 10.9 ± 12.0), with an average paired difference of + 66.2 g, which based on the Wilcoxon signed-rank test, was not statistically significant. For the young adult, he had an LV mass of 835.1 g at baseline and 789.1 g at follow-up after six months. Mean GLS at baseline for the pediatric patients was − 14.1% ± 2.9% which is lower than the − 20% measured in children without any cardiomyopathy [20]. Four pediatric patients (50%, n = 4) had follow-up GLS values, of which two had a GLS that was less negative (50%, n = 2). Mean GLS at last follow-up was − 14.7% ± 5.1%, with an average paired difference of + 0.14%. For the adult patient, the GLS at baseline was − 5.8% and at last follow-up was − 3.0%. Of note, this patient’s LVEF was already less than 50% base baseline. All echocardiogram data can be seen in Table 4; Fig. 5, and Supplementary Fig. 1.
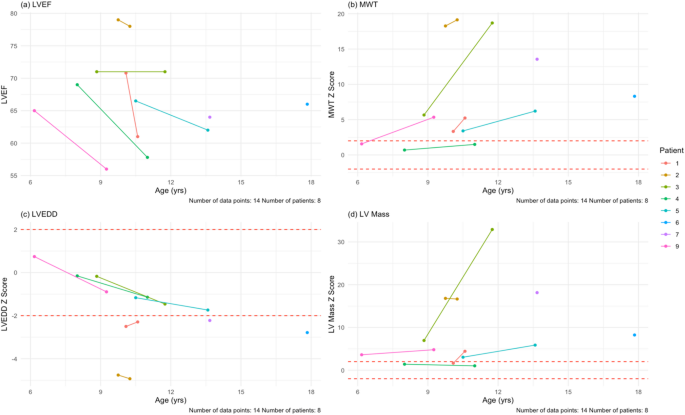
Echocardiogram findings for the pediatric patients in the cohort at their baseline and last follow-up visits. (a) LVEF plotted against age in our cohort. There is a downward trend (blue line) as age increases. (b) MWT z-scores plotted against age in our cohort. DD hearts thicken over time, as in this cohort. (c) LVEDD z-scores plotted against age. There is a downward trend in this age range. (d) LV mass z-scores plotted against age in our cohort. Despite the hearts having more mass, the scores remain relatively stable over time. The mean follow-up time for echocardiogram assessment was 24.5 months. MWT = maximum wall thickness, LVEDD = left ventricular end-diastolic dimension
DD causes dysfunctional macroautophagy that results in accumulation of intracellular vacuoles, a mismatch between supply and demand of energy within cells and eventual cell death. The cardiac hypertrophy and fibrosis that occur result in significant electrophysiologic abnormalities including high voltage with repolarization abnormalities, conduction disorders, accessory pathways, and atrial and ventricular arrhythmias. Therefore, electrocardiogram (ECG) findings at baseline were also assessed in all nine patients. Baseline ECG findings included: Wolff-Parkinson-White (WPW) (56%, n = 5), LV hypertrophy (44%, n = 4), biventricular hypertrophy (22%, n = 1), sinus bradycardia (11%, n = 1), and 1st degree atrioventricular (AV) block (11%, n = 1).
Most of our patients (89%, n = 8) had respiratory related problems: wheezing (44%, n = 4), dyspnea (33%, n = 3), cough (22%, n = 2), asthma allergies (11%, n = 1), and sleep apnea (11%, n = 1). In our cohort, seven patients underwent PFTs at baseline (78%, n = 7) and five patients completed a follow-up visit (71%, n = 5). At baseline, the average percent of predicted seated upright FVC was 74.6% ± 8.5% (2.3 ± 1.3 L) (Table 5). After a mean follow-up time of 5.8 months, one patient had a lower upright FVC (20%, n = 1), one had no change (20%, n = 1), and three had an increase (60%, n = 3). The mean percent of predicted upright FVC at last follow-up was 74.0% ± 9.6% (2.4 ± 1.6 L).
Mean percent of predicted supine FVC at baseline was 60.7% ± 12.6% (1.8 ± 1.2 L). After the mean follow-up time, from the five patients who completed a follow-up visit, one patient had a lower FVC (20%, n = 1), one had no change (20%, n = 1), and the rest had an increase (60%, n = 3). Last follow-up percent of predicted supine FVC was 64.4% ± 14.0% (2.0 ± 1.3 L).
The mean percent of predicted FEV1 was 53.6% ± 16.6% (1.6 ± 1.0 L). At follow-up, two patients had a lower percent of predicted FEV1 (40%, n = 2). The mean percent of predicted FEV1 at follow-up was 61.6% ± 16.8% (1.8 ± 1.3 L).
For the FEV1/FVC ratio, the mean percent of predicted was 103.4% ± 1.6% (0.89 ± 0.09). At follow-up, one patient had a lower percent of predicted FEV1/FVC (20%, n = 1), one had no change (20%, n = 1), and three had an increased (60%, n = 3). The mean at follow-up was 108.2% ± 7.7% (0.92 ± 0.05 L).
In this study, CPET was performed at baseline and at one follow-up visit, with a mean follow-up time of 5.2 months. Five patients performed CPET in our cohort (56%, n = 5) at baseline and at one follow-up, for a total of 10 visits. Our cohort had a baseline mean VO2 max of 17.2 ± 4.4 ml/kg/min (percent of predicted of 39.0 ± 9.0%) (Table 6; Fig. 6a, Supplementary Table 6). After a mean follow-up time of 5.2 months, three patients had a lower VO2 max (60%, n = 3). At last follow-up, the mean VO2 max was 17.6 ± 5.6 ml/kg/min (percent of predicted of 42.6 ± 13.0%). Out of the 10 total visits, six assessments reached an RQ of ≥ 1.0 (60%, n = 6). The baseline mean for ventilatory efficiency (VE/VCO2 slope) was 28.4 ± 2.5 (Table 6; Fig. 6b, Supplementary Table 6). After the mean follow-up time, two patients had a smaller VE/VCO2 slope (40%, n = 2). At last follow-up, the mean VE/VCO2 slope was 29.5 ± 3.2.
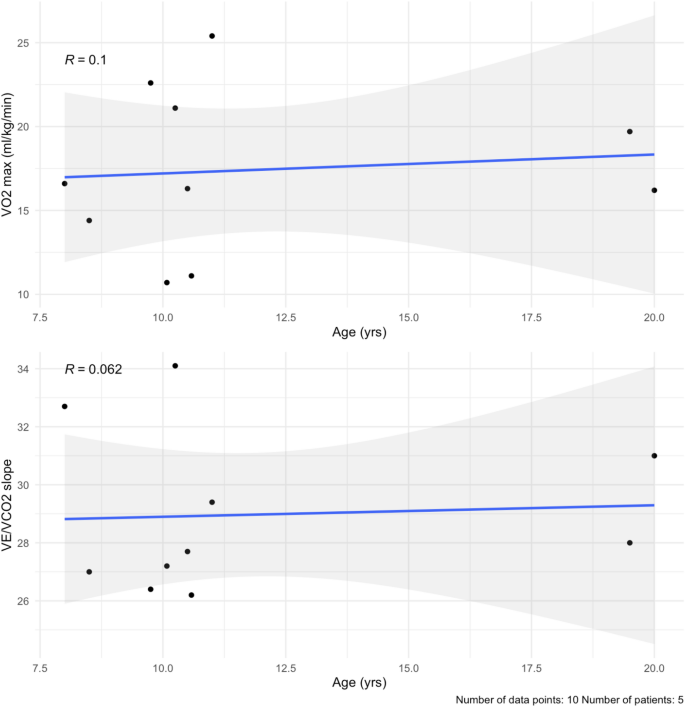
(a) VO2 max by age. There is a small positive correlation with age (blue line), likely due to lung maturity during puberty. (b) VE/VCO2 slope values plotted over time in the cohort. They remain stable over the 6 months. The mean follow-up time was 5.2 months
Seven patients (78%) in our cohort reported neuromuscular symptoms at baseline: delay in motor milestones (67%, n = 6), difficulty running (67%, n = 6), difficulty walking (67%, n = 6), fatigue (67%, n = 6), weakness (67%, n = 6), joint pain (33%, n = 3), difficulty sitting (11%, n = 1), hypotonia (11%, n = 1), loss of range of motion (11%, n = 1), and abnormal gait (11%, n = 1). All limbs were found to be affected across the cohort: lower proximal limb (78%, n = 7), lower distal limb (67%, n = 6), upper proximal limb (56%, n = 5), upper distal limb (44%, n = 4).
For the 6MWT, all patients had baseline testing (100%, n = 9), but only seven patients had follow-up testing (78%, n = 7). At baseline, our cohort averaged 448.7 ± 64.7 m (Table 6; Fig. 7, Supplementary Table 7). After a mean follow-up time of 23.3 months, four patients walked shorter distances (57%, n = 4). The mean distance at last follow-up was 405.9 ± 136.1 m, with an average paired difference of -25.9 m.
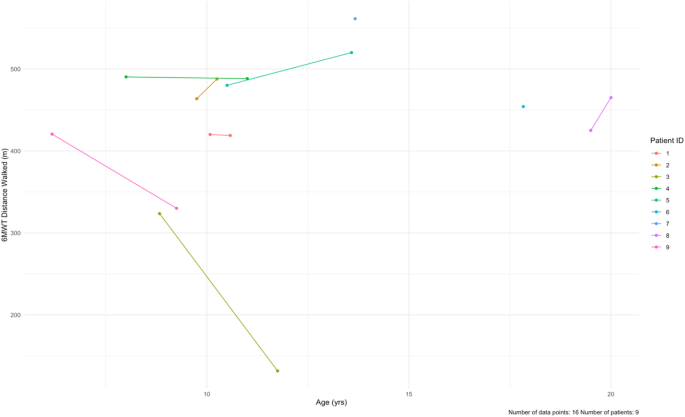
Longitudinal data for the 6-minute walk test (6MWT) at baseline and at follow-up for each patient in the cohort. The data is distance walked (meters) by age. Patients 6 and 7 only had baseline data. The mean follow-up time was 23.3 months
The results for the NSAA, 10MWT, time to rise from floor, and 4SC tests are shown in Fig. 8. For the NSAA, all patients completed the test at baseline (100%, n = 9). Seven patients completed follow-up visits (78%, n = 7), with a mean follow-up time of 23.1 months. The average NSAA total score at baseline was 28 ± 9 out of a max total of 34. After the follow-up time, one patient had a worse NSAA total score (14%, n = 1) and two patients had better scores (29%, n = 2) (Supplementary Table 8, Supplementary Fig. 4a). Out of the seven patients who had follow-up data, four (57%, n = 4) had no changes over 6-, 12-, 24-, and 36-month follow-ups. One of our patients, Patient 3, had markedly worse NSAA total score and 10MWT and 4SC times. Notably this patient had difficulty following instructions, which may have impacted these scores (Supplementary Fig. 4).
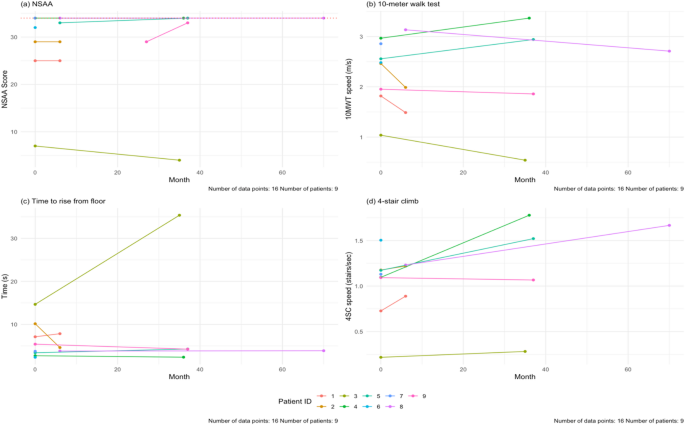
(a) Baseline and last follow-up NSAA scores for the patients in the cohort. The maximum score is 34 (dashed line). The NSAA trended up with age (blue line). (b) Baseline and last follow-up speed during the 10MWT for our cohort. There is a positive correlation with age. (c) Baseline and last follow-up speed during the 4SC in our cohort. There is a positive correlation with age. The mean follow-up time for all these tests is 23.1 months. NSAA = North Star Ambulatory Assessment, 10MWT = 10-meter walk test, 4SC = 4-stair climb test
For the 10MWT, all nine patients (100%, n = 9) completed the test at baseline, but only seven (78%, n = 7) patients had follow-up testing done. The mean follow-up time was 19.4 months. In our cohort, ages 6–20, the baseline average speed was at 2.4 ± 0.6 m/s. At the end of study, two patients were slower (29%, n = 2). The average speed at follow-up was 2.2 ± 1.0 m/s.
For the time to rise from floor test, nine patients completed the test at baseline (100%, n = 9) and seven patients completed follow-up testing (78%, n = 7). The mean follow-up time was 23.3 months. The average time taken at baseline was 5.9 ± 4.1 s. At the end of the study, three patients took longer to rise from the floor (43%, n = 3). The mean time at last follow-up was 8.9 ± 11.8 s.
For the 4SC, we had nine patients complete the test at baseline (100%, n = 9), and seven patients with follow-up visits (78%, n = 7). The average speed at baseline was 1.1 ± 0.4 stairs/sec. After the mean follow-up time of 19.4 months, two patients were slower (29%, n = 2) (Fig. 8d).
All patients completed the baseline laboratory measurements and follow-up with a mean follow-up time of 18.6 months. Of note, cardiac biomarkers including N-terminal pro brain natriuretic peptide (NT-proBNP) and high sensitivity cardiac troponin T (hs-cTnT) were elevated at both baseline and follow-up. Similarly, skeletal biomarkers including CPK and aldolase were similarly elevated at baseline and follow-up, as were AST, ALT and LDH (Table 7). Figure 9 shows the values for NT-proBNP and hs-cTnT for all patients at baseline and follow-up only, where mean paired difference was 971.3 pg/mL and 37.3 respectively, which based on Wilcoxon signed-rank test was a significant increase in NT-proBNP, but not hs-cTnT; Supplementary Fig. 5 shows the values at all visits.
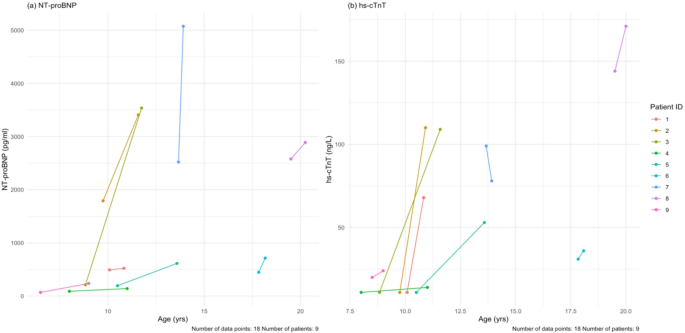
(a) Baseline and last follow-up NT-proBNP values for each patient. (b) Baseline and last follow-up hs-cTnT values for each patient. The mean follow-up time for all these tests is 18.7 months. NT-proBNP = N-terminal pro brain natriuretic peptide, hs-cTnT = high sensitivity cardiac troponin T

 Getty Images
Getty ImagesWork to redevelop Glasgow’s George Square will not be “accelerated” to get it ready for the Commonwealth Games next year, the council has said.
The square is…

When landowner and hunter James Barkhurst went scouting his property about a month ago to assess the local deer population ahead of the fall hunting season, he was left in shock.
“I’ve seen about 14 dead in less than a mile stretch. There’s…